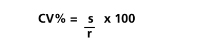Qualitative Analysis-Finding out more and more about the nature and
composition of materials – both natural and synthetic – and how these materials behave towards each other.
Quantitative Analysis- the emphasis was directed to determining how much of
a particular component (or group of components) is present.
Classical Methods:
Gravimetric analysis: converting the analyte into an insoluble form (precipitate) which can be filtered, dried and weighed.
Volumetric analysis: use of standard solutions (known volumes of known concentrations) to react with the analyte in a titration to a defined ‘end point’, e.g., a colour change.
Electrical methods: based on electrochemistry and may involve measurement of current, voltage, resistance or conductance. pH is perhaps the best known here.
Instrumental Methods:
- Spectroscopy
- Chromatography
- Mass Spectroscopy
- NMR
Separative & Non Separative Methods

common steps in an analytical sequence used to determine an analyte.
Some complex samples may require additional pre-treatment (i.e. filtration, precipitation, centrifugation and extracting the analyte with an organic solvent) or by removing moisture or dissolving the sample in an appropriate solvent.
In quantitative analysis: the more steps involved in the analytical sequence, the greater the chance of losing some of the analyte. Therefore it is wise to determine the ‘recovery’ of the analyte after a particular stage. This is carried out by subjecting samples with a known concentration of the analyte to the pre-treatment, and then
determining whether the pre-treatment has affected the quantity of analyte originally present.
Where possible, a concentration stage enables the analyst to obtain the maximum amount of information from the analyte during the actual analytical stage, or enables the analyst to carry out duplicate measurements
This is the stage which constitutes the ‘measurement’ of the analyte. In
modern instrumental techniques, this stage may well be carried out automatically.
The interpretations of the results obtained depend upon the nature of the analyte and the design of the analytical procedure. In modern instrumental methods, the results obtained relate to an electrical signal generated by some particular property of the analyte. This electrical signal needs to be interpreted by determining the effect produced by known amounts of the analyte, in a process called standardisation. The results may well be subjected to statistical analysis and their significance interpreted in the light of experience about the techniques employed.
A knowledge of the “correctness” or reliability of analytical methods will enable the analytical chemist to select the most appropriate technique for the job in hand. All analytical methods are susceptible to the presence of errors.
Sources of errors:
- Systematic Errors (Determinate)
- Random Errors (Indeterminate)
- Gross Errors (Total)
Definitions
Systematic Errors
These errors may arise because the analyst does not follow the accepted method exactly, uses non-specified or impure reagents, fails to allow reactions to proceed to completion or relies on instruments that are
faulty, poorly maintained or non-calibrated. A number of techniques can be adopted to determine
their influence such as use of control samples containing a known quantity of analyte, comparing the results of different, but appropriate, methods, or subtracting the value of blank samples (a sample known to contain all components except the analyte).
Random Errors
They may arise through
slight variations between different batches of reagents, slight temperature changes during a reaction or different analysts identifying a different end-point (such as a colour change) in a reaction. Generally, the effect of random errors on a result is small and in a set of replicate analyses the statistical mean value will correspond to the true value. That is to say, if an analysis is repeated a number of times; some of the results will be lower than the true value and some will be higher. If
the average result is calculated high and low results will cancel each other out and the average result (statistical mean) will correspond to the true value.
Gross Errors
Gross errors tend to make the final result meaningless. They may be caused by selecting an entirely inappropriate method, eliminating a key stage or using an instrument
incorrectly or one that is faulty. Their effect is to give rise to wide variations in replicate results and they should not be subjected to statistical analysis in order to provide a reliable result. The analyst cannot be sure that replicate results will be evenly distributed above and below the true value.
Accurate
An accurate result is one in which systematic errors are small and the average result or measurement
of a set of replicates equals, or is very close to the true result.

Absolute error Ea: a set of replicate results (r) and the true value (Tv)

Relative error
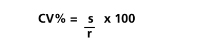
Measure of precision CV: average deviation (s), average value of the results (r)
Analytical chemists need to obtain both accurate and precise results which can be done with good laboratory practice to reduce systematic errors and by carrying out replicate analyses to reduce random errors and improve precision
Major separative analytical method
General Principles:
- The term chromatography: universal for describing the separation of a mixture of solutes dissolved in a common solvent by allowing the mixture to interact with two, discrete phases.
- Stationary and mobile phases: Stationary phase could be solid or a liquid (that remains stationary) by being chemically bonded to an inert support. Mobile phase may be a liquid or a gas
- The solute interacts with the stationar phase in four ways:
- Partition- solute dissolves in the stationary phase and moves through as if in solution
- Adsorption or affinity- solute is adsorbed by the stationary phase and moves through the solid particles by surface effects
- Ion exchange- solute chemically reacts with the stationary phase or with ions attached to it
- Gel permeation or size exclusion- Porous stationary phase allows the smallest solute molecules to permeate and larger molecules will be washed through rapidly. (Stationary phase is usually a gel). Separating the largest molecules first!

Use of Chromatography to Screen Raw Materials:
Chromatography is regularly used to check the purity of raw materials and to compare results with pure samples.
Gas-liquid chromatography (GLC) and gas-solid Chromatography (GSC) comprise this. For GLC, the stationary phase is a high boiling point liquid chemically bonded to an inert support and the process of separating components occurs predominantly by partition. In GSC, the stationary phase is a solid and separating occurs by adsorption. Components in this method separate due to differences in boiling points, solubility or adsorption.
GLC is a rapid technique for the separation of complex mixtures and very small samples. This method is suitable for qualitative and quantitative analysis
Qualitative Analysis: The retention time of components of a sample can be compared directly with those of known standards
Quantitative Analysis: This revolves around the methods of estimating the magnitude or area of the peak.
HPLC is the most powerful chromatographic technique. Substances with highly polar or ionisable functional groups often show poor chromatographic behaviour by GC, being very prone to tailing. Thus, HPLC is the better method for macromolecules, inorganic or other ionic species, labile natural products, pharmaceutical compounds and biochemicals. Applications include separation of proteins, amino acids, hydrocarbons, fatty acids, antibiotics, drugs, steroids
Increasing the surface area of the stationary phase by reducing particle size improves resolution of the column but below a certain particle size, its difficult for the solvent to flow through. HPLC overcomes this problem by using a pump to force the mobile phase through the column packing. This must be capable of maintaining a constant pressure, without pulsing.
In GC, increasing the column temperature can speed up separation process. In HPLC this is achieved by changing the solvent composition as the extraction is run (i.e creating a concentration gradient in the mobile phase).
Spectrometry: describes a range of similar techniques which measure the amount of electro-magnetic radiation absorbed or emitted by an analyte.
Spectrometry is a non-separative method and therefore to be efficient, the analyte must be ‘presented’ to the electromagnetic radiation either in a pure state or in a form where other compounds are unable
to interfere.
Spectrometry can provide information of a qualitative and a quantitative nature and besides discrete analysis, spectrometric methods may be combined with other analytical methods.
The absorption or emission of electromagnetic radiation by the analyte is related to the atomic and/or molecular structure and may be caused by electron movements between energy levels, vibrations between atoms in molecular or functional groups, and even changes to the ‘spin’ of nucleii.
The various types of spectrometric methods :
• fluorescence spectrometry (fluorescent light emitted by the analyte)
• nuclear magnetic resonance (NMR) (nucleii resonate in a magnetic field)
• infrared (IR) spectrometry (infrared region of the spectrum – felt as heat)
• UV/visible spectrometry (that region of the spectrum that appears as visible light)
EM radiation is defined mathematically by quite complex equations representing a combination of magnetic and electrical vectors. For convenience, EM is described as an electrical ‘wave’ . All EM radiation is transmitted at the same speed (3 x 108 m/s) and may be described in terms of wavelength, wave number or frequency.
UV/VIS spectrophotometry involves analysis of samples using EM radiation in the region which is detected by the human eye - the visible region (400 nm – 800 nm) and also the ultraviolet region
(100 nm- 400 nm). Generally, a wide range of compounds can absorb
radiation of this energy-absorptiometry. A wide range of organic, metallic, and biochemicals can absorb in this EM region. The specific wavelength wher absorption is strongest gives the analyst information about the structure of molecules, whilst the amount of radiation absorbed gives a measurement of concentration.
When UV/visible radiation is directed through a solution contained in a cuvette, a portion of the light is reflected (Ir), a portion is absorbed (Ia) within the medium and the remainder is transmitted (It).